GROUP DYMAMICS
Lidong Feng a, b, Songyang Feng c, Xinchao Bian a, b, **, Gao Li a, b, Xuesi Chen a, b, *Polymer Degradation and Stability 157 (2018) 212e223
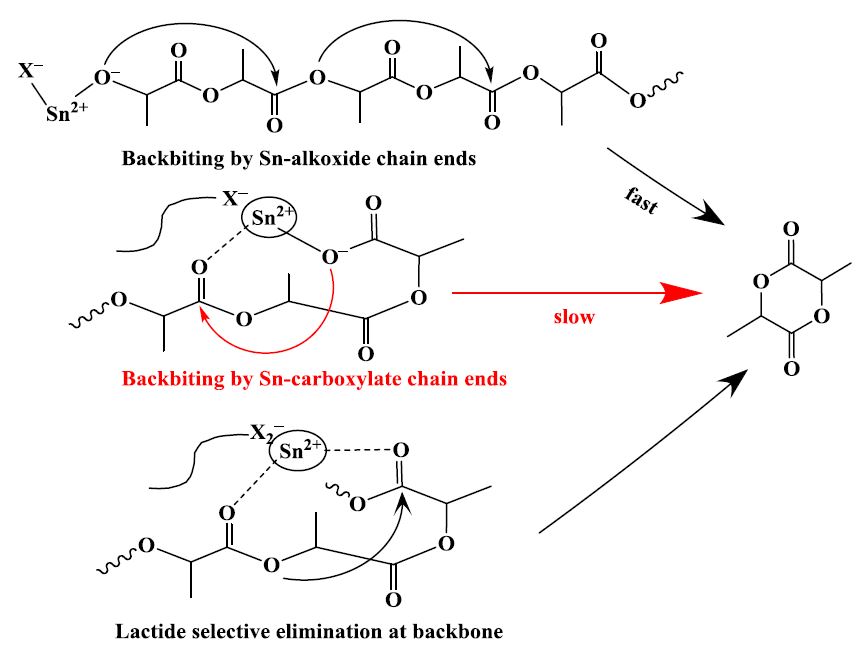
In order to obtain the mechanisms of the pyrolysis reaction that poly (lactic acid) (PLA) gives lactide in the presence of Sn, the four types of low molecular weight PLAs (L-PLA) with different end-groups were prepared, including HO-L-PLA-COOH, R-L-PLA-OH, R-L-PLA-COOH, and R-L-PLA-R, and depolymerized by thermogravimetry at different heating rates. As for the pyrolysis reactions of the L-PLAs, their activation energy (Ea) were estimated by the several isoconversional model-free methods and their kinetic models were also explored by the Malek method. The random degradation behavior of L-PLAs was determined by the plots of lnf ln½1 ð1 wÞ0:5g vs. 1/T for experimental data from TG for L-PLAs and model reactions. The experimental results showed that the Ea values and the possible kinetic models for the pyrolysis reactions of the L-PLAs were different due to the different end-groups and thereof the mechanisms for the pyrolysis reactions of PLA that give lactide were proposed under the catalysis of Sn. The pyrolysis reactions of R-L-PLA-OH and R-L-PLA-R trend to be controlled by a single kinetic model. The pyrolysis reactions of HO-L-PLA-COOH and R-L-PLA-COOH are more complex and controlled by not less than two kinetic processes. The pyrolysis reaction of PLA selectively produces lactide through the unzipping and intramolecular transesterification reactions, including the backbiting reaction caused by the Sn-carboxylate and Sn-alkoxide chain ends, which are directly formed from the hydroxyl and carboxyl end-groups of PLA, and the lactide selective elimination at the random position of the polymeric backbone.